The safety of PALYNZIQ® Injection has been studied in the phase 3 PRISM clinical trial programme1,2
All 261 patients experienced at least one adverse reaction, the majority of which were mild to moderate, with ISR, arthralgia and hypersensitivity reactions reported most frequently1,3
- In clinical trials, adverse reaction rates were highest during induction and titration and decreased over time3
Adverse reactions in patients treated with PALYNZIQ®3*†‡
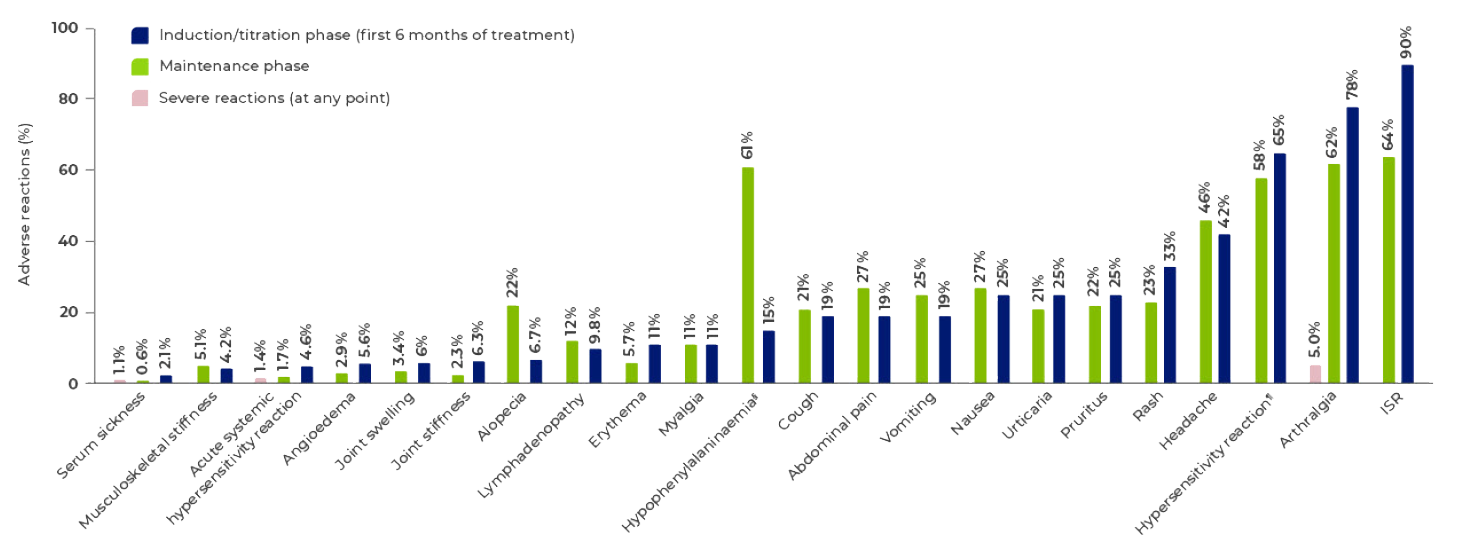
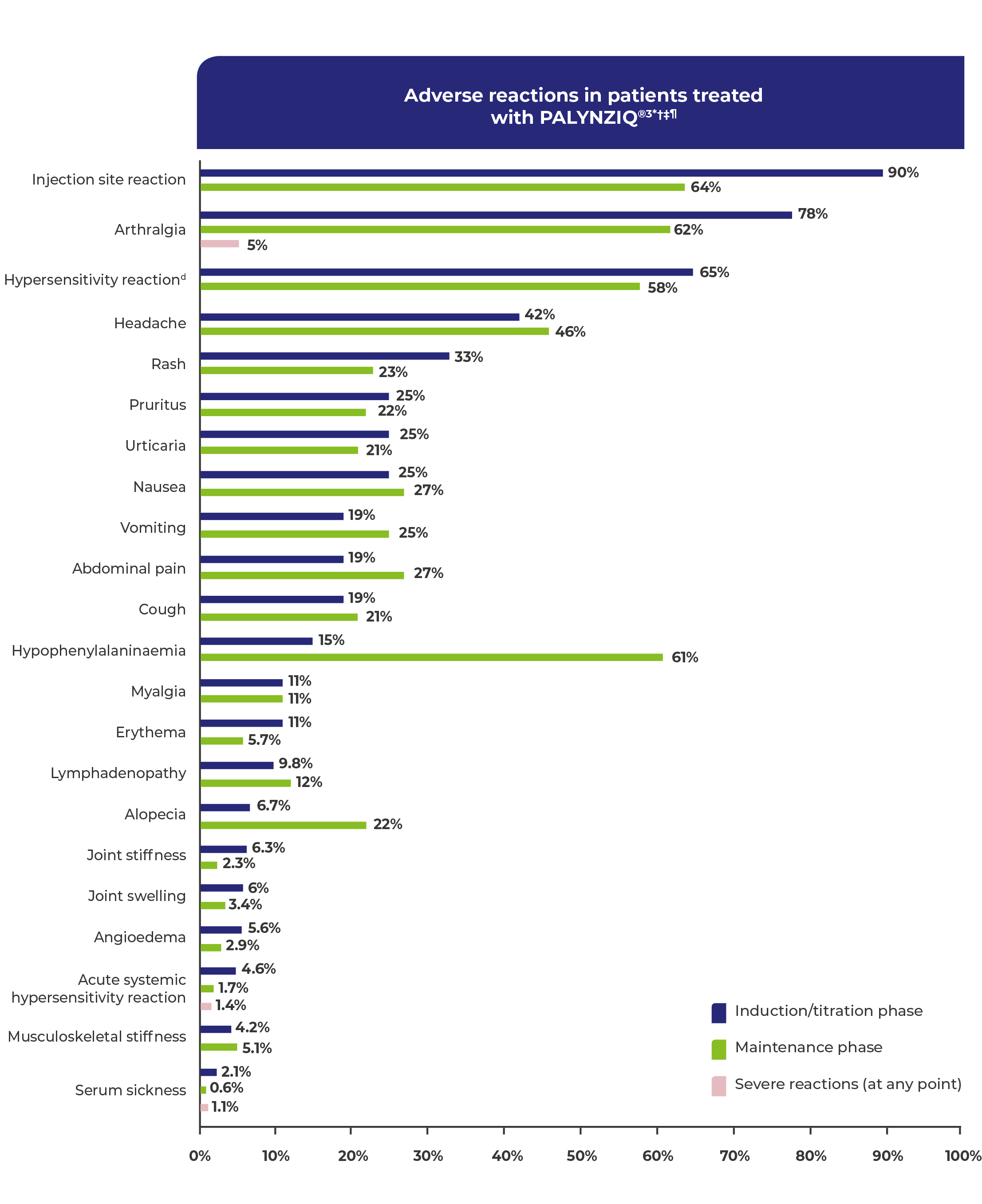
Adverse reactions occurring in ≥5% of patients treated with PALYNZIQ® and patients reporting severe reactions.1
Includes all patients who followed an induction, titration, and maintenance dosing regimen in phase 2 and phase 3 studies (N=285).3
Induction and titration phase reflects the time prior to reaching blood Phe levels <600 μmol/L while on a stable dose (ie, maintenance dose).3
Hypersensitivity reactions cover a group of terms including acute systemic hypersensitivity reactions and can manifest as a range of symptoms including angioedema, serum sickness, rash, and urticaria. Acute systemic hypersensitivity reactions occurred in 6% of patients during clinical trials.3
Non-IgE-mediated acute hypersensitivity reactions were observed in phase 3 clinical trials3
- In clinical trials with an induction/titration/maintenance regimen, 6% of patients (16 of 285) experienced a total of 25 hypersensitivity reactions3
- No drug-specific IgE was detected at or near the time of the episodes3
- 10 of 16 (63%) patients who experienced an acute hypersensitivity reaction were rechallenged, of which four had at least one recurrence3
- Most episodes occurred within 1 hour after injection (88%, 22 of 25 episodes), though delayed episodes also occurred up to 24 hours after administration3
- Acute hypersensitivity reactions occurred most frequently in the induction and titration phases, and were reduced 7-fold in the maintenance phase3
Management of anaphylaxis in clinical trials3
- 44% of acute hypersensitivity reaction episodes (11 of 25) were managed with administration of auto-injectable epinephrine
All acute hypersensitivity reactions resolved without sequelae3
Type III immune complex–mediated hypersensitivity reactions
- Hypersensitivity reactions occurred in 75% of patients (213 of 285) treated with PALYNZIQ®3
- The rate of hypersensitivity reactions was highest during the induction/titration phases and decreased during the maintenance phase, but reactions can occur at any time3
- In clinical trials, hypersensitivity reactions were managed with dose reduction, treatment interruption, treatment withdrawal, and/or concomitant medicinal products3
Strategies for managing potential acute hypersensitivity reactions
Requirements
- Prescribe auto-injectable adrenaline to all patients treated with PALYNZIQ®. Prior to the first dose, instruct the patient and adult observer (if applicable) on how to recognise the signs and symptoms of acute hypersensitivity reaction, on how to properly administer auto-injectable adrenaline, and to seek immediate medical care upon its use3
- Instruct patients to carry auto-injectable adrenaline with them at all times during treatment with PALYNZIQ®3
Considerations
- For at least the first 6 months of treatment, when the patient is self-injecting, an observer must be present for at least 1 hour after PALYNZIQ® administration3
- The observer should be able to recognise the signs and symptoms of an acute hypersensitivity reaction, know to seek immediate medical care if a reaction occurs, and be able to administer auto-injectable adrenaline3
- Premedication is required prior to each dose of PALYNZIQ® during induction and titration, and may be considered during maintenance based on individual tolerability:3
- H1-receptor antagonist
- H2-receptor antagonist
- Antipyretic
References: 1. Thomas J et al. Mol Genet Metab 2018;124(1):27–38. 2. Harding CO et al. Mol Genet Metab 2018:124(1):20–26 3. PALYNZIQ® Summary of Product Characteristics.