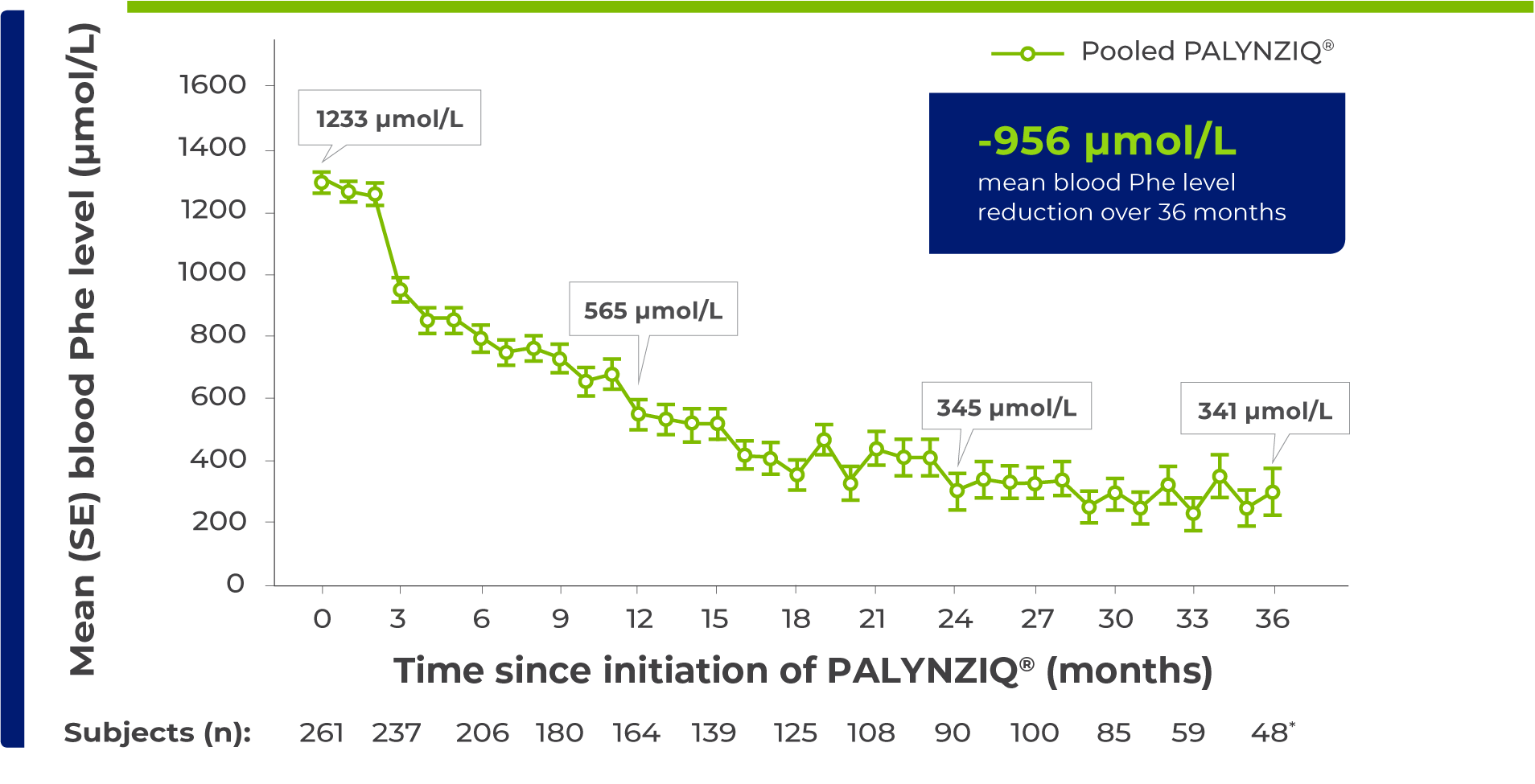
PALYNZIQ® Injection maintained blood Phe level reductions over 36 months1
Mean (SE) blood Phe levels over time1
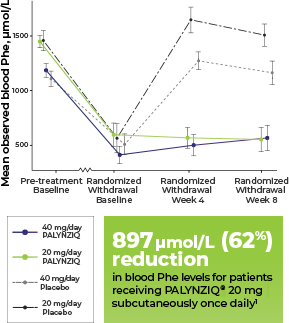
Patients in the RDT (n=86) had been randomised to receive PALYNZIQ®
PALYNZIQ® achieved statistically significant reduction in blood Phe levels at 8 weeks vs placebo in the pivotal efficacy study (primary endpoint; P<0.0001)1
Long-term treatment with PALYNZIQ® resulted in reductions in blood Phe levels that were associated with above the minimally clinically important difference improvements in neurocognitive and neuropsychiatric symptoms1
PALYNZIQ® delivered a 72% mean blood Phe level reduction from baseline over 36 months1
Reflects number of patients who reached month 36 of treatment at the time of the data analysis and had a scheduled Phe assessment for month 36.1
References: 1. PALYNZIQ® Summary of Product Characteristics. 2. Harding CO et al. Mol Genet Metab 2018;124(1):20–26. 3. Thomas J et al. Mol Genet Metab 2018;124(1):27–38.