PALYNZIQ® Injection is a daily at-home subcutaneous injection available in 3 dosage strengths1
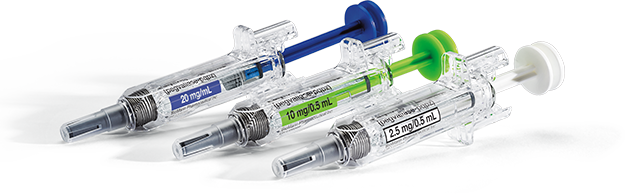
PALYNZIQ® is available in 3 different dosage strengths:1
2.5 mg /0.5 mL single-dose prefilled syringe (white)10 mg /0.5 mL single-dose prefilled syringe (green)20 mg/mL single-dose prefilled syringe (blue)
- PALYNZIQ® is supplied in a prefilled syringe intended for use as a single at-home subcutaneous injection1
- PALYNZIQ® should be refrigerated at 2ºC to 8ºC1
- PALYNZIQ® can be safely stored at room temperature in the original carton for up to 30 days1
- Do not re-refrigerate once stored at room temperature (below 25°C)
- Do not freeze
Recommended dosing regimen
- There are 3 phases to the recommended dosing regimen for PALYNZIQ®: induction, titration, and maintenance1
- The maximum daily dose of PALYNZIQ® is 60 mg1
The first injection must be performed under the supervision of a healthcare provider and patients must be closely observed for at least 1 hour following injection. Prior to self-injection, the patient's competency with self-administration must be confirmed.1
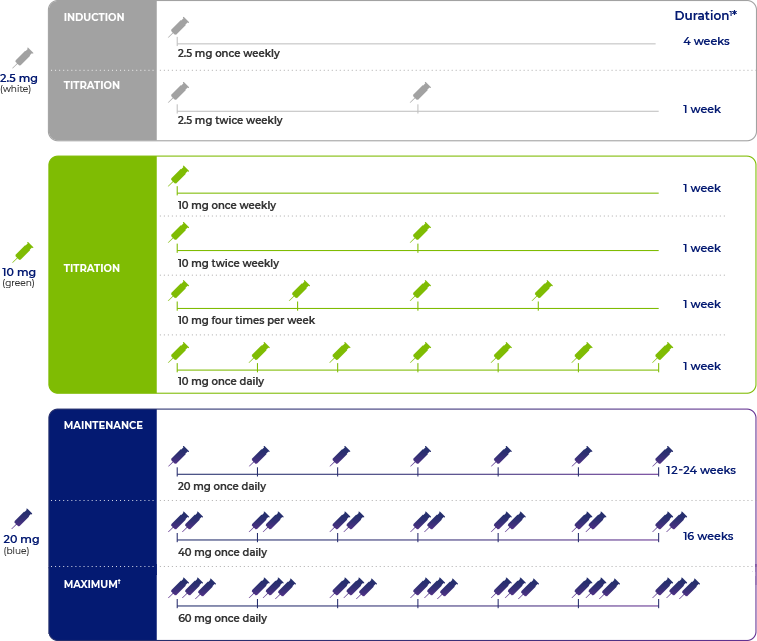
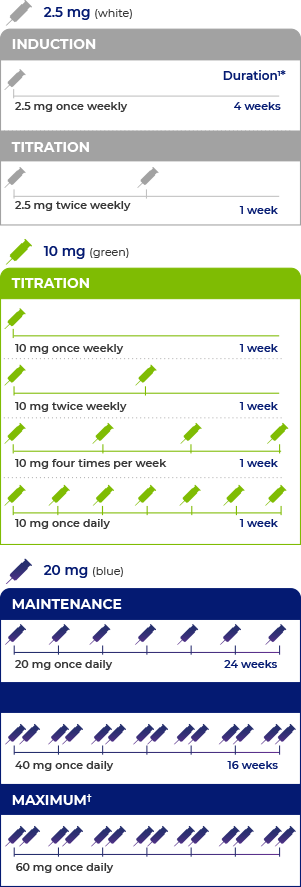
Additional time may be required between dose escalations based on patient tolerability.1
Individualise treatment achieve blood Phe levels of 120–600 µmol/L. Consider increasing to a maximum of
Therapeutic dosage is based on patient tolerability, blood Phe concentrations, and dietary Phe intake1
In clinical trials, consistent dietary Phe intake was recommended until therapeutic dosage was achieved2
- Before initiating treatment, blood Phe levels must be obtained. Monitoring of blood phenylalanine level is recommended once a month1
- Dietary Phe intake should remain consistent until a maintenance dosage is established1
- Most patients were not on a Phe-restricted diet prior to and during the trials2
- Patients’ total protein intake remained relatively stable throughout treatment, while dietary Phe intake increased2
- If blood Phe levels are below 30 µmol/L, dietary protein intake should be increased to appropriate levels, and then, if needed, the dosage of PALYNZIQ® should be reduced1
A 20% reduction in blood Phe considered the first sign of response in clinical trials3
- Treatment may be continued to reach individualised Phe targets once this threshold is met, based on patient tolerability1
References: 1. PALYNZIQ® Summary of Product Characteristics. 2. Thomas J et al. Mol Genet Metab 2018;124(1):27–38. 3. Harding CO et al. Mol Genet Metab 2018;124(1):20–26.