You can view the dosing schedule here.
ACMG, American College of Medical Genetics and Genomics.
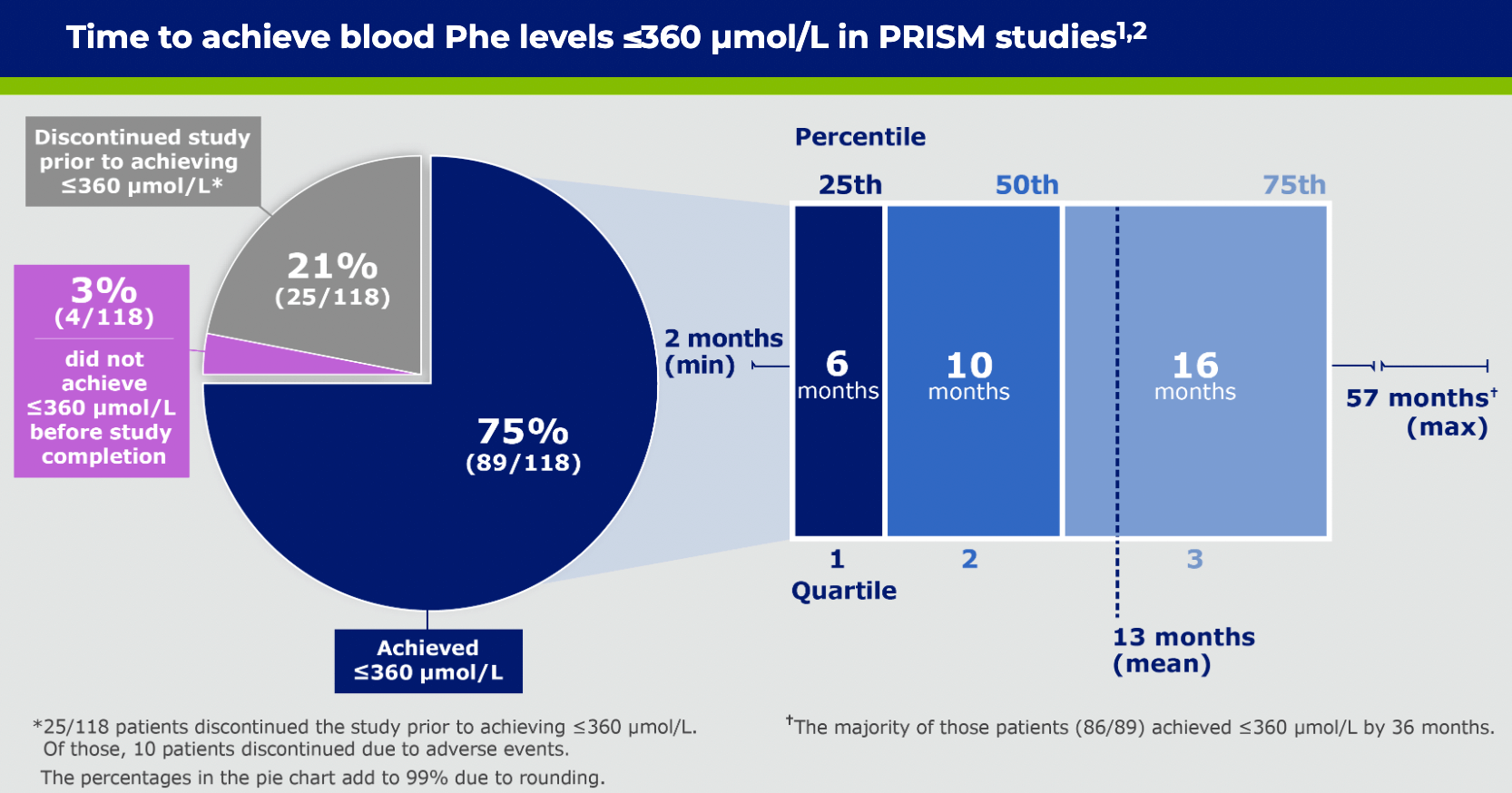
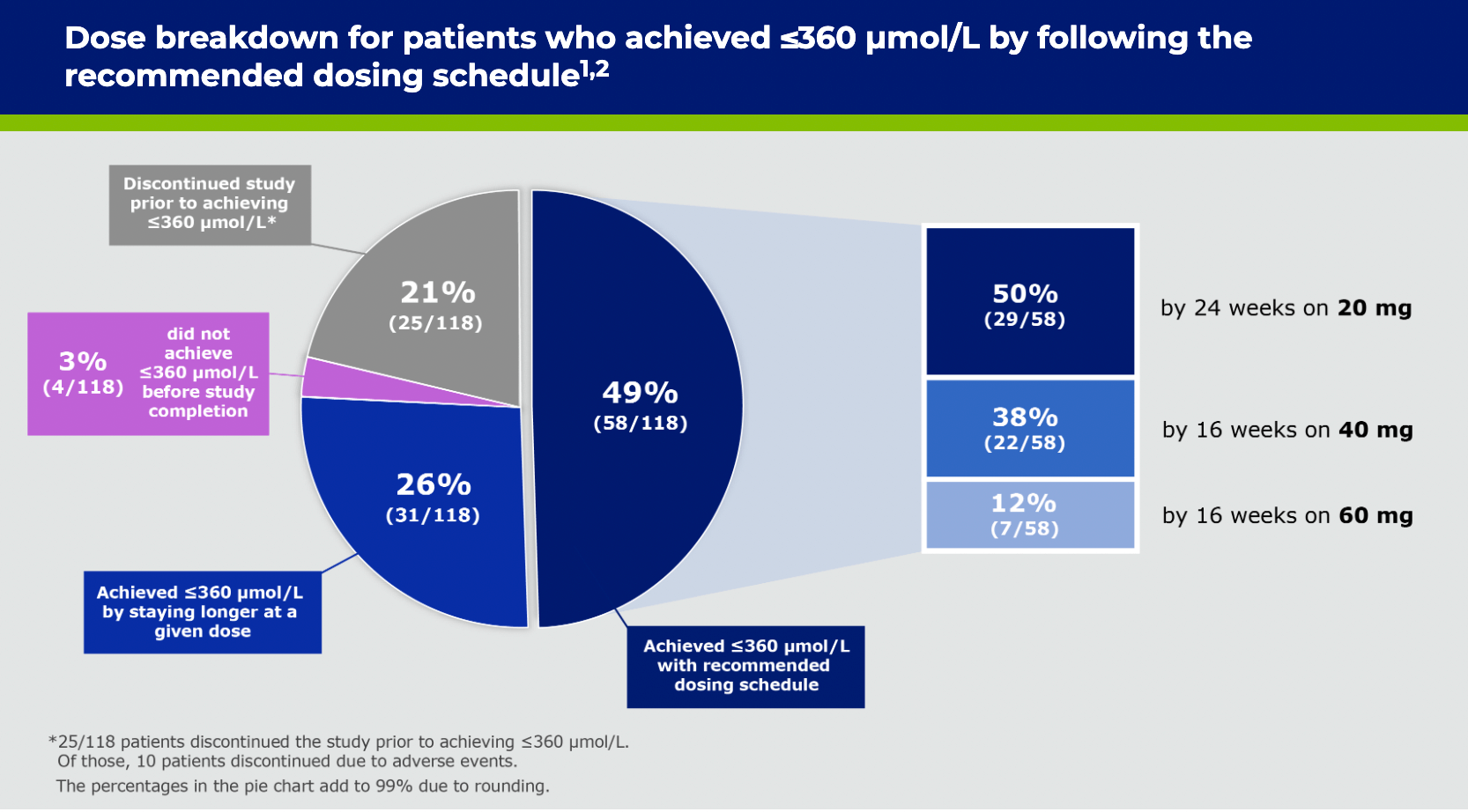
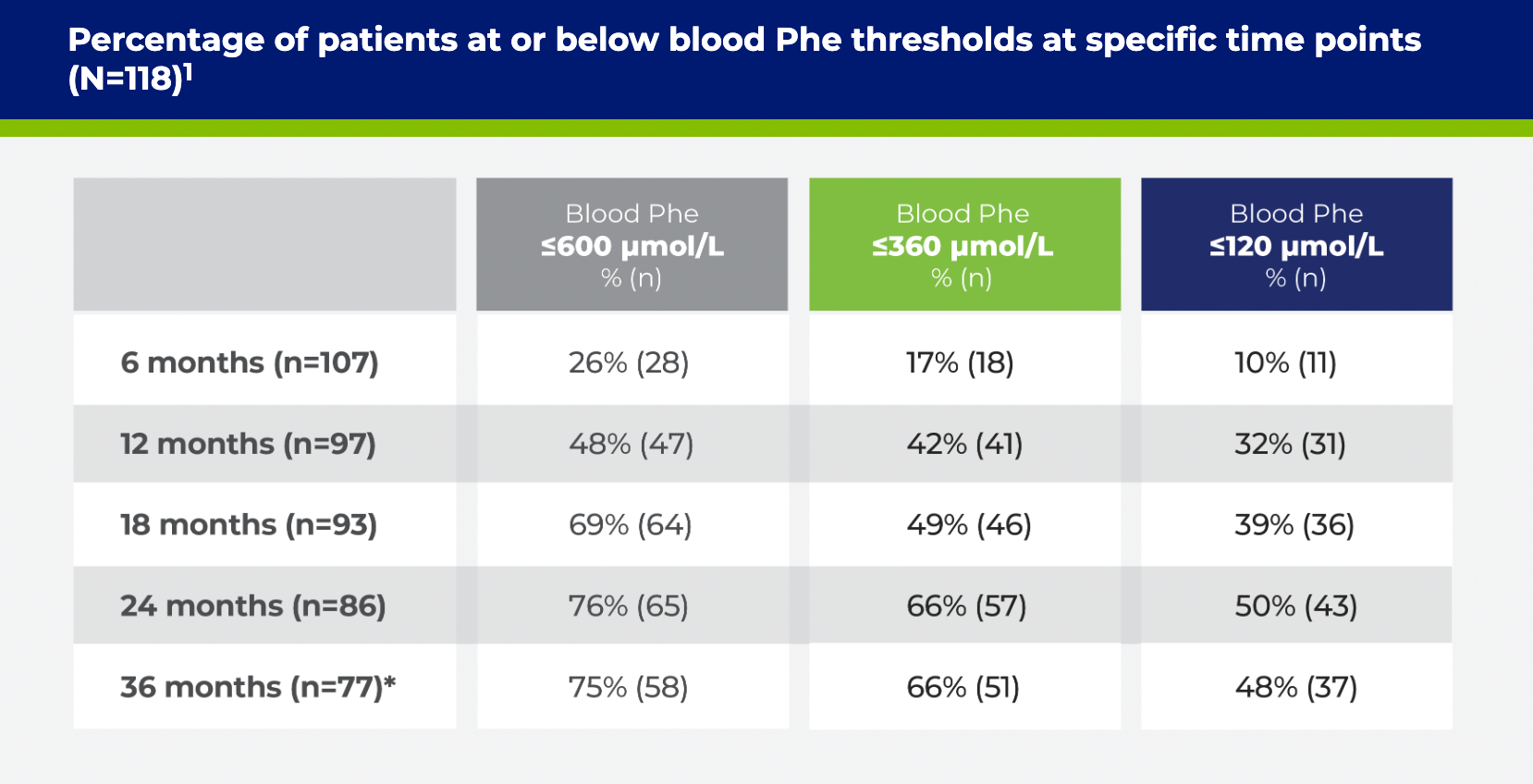
*Changes in sample size are due to patients’ Phe levels not being collected at all time points and subjects terminating from study early.2
*Patients who remained in the trial.1
BOXED WARNING: RISK OF ANAPHYLAXIS
WARNINGS AND PRECAUTIONS
Anaphylaxis
Other Hypersensitivity Reactions
ADVERSE REACTIONS
Blood Phenylalanine Monitoring and Diet
DRUG INTERACTIONS
Effect of PALYNZIQ on Other PEGylated Products
USE IN SPECIFIC POPULATIONS
Pregnancy and Lactation
Pediatric Use
Geriatric Use
You are encouraged to report suspected adverse reactions to BioMarin at 1-866-906-6100, or to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, with Boxed Warning for risk of anaphylaxis, and Medication Guide here.
INDICATION
PALYNZIQ is a phenylalanine (Phe)-metabolizing enzyme indicated to reduce blood Phe concentrations in adult patients with phenylketonuria who have uncontrolled blood Phe concentrations greater than 600 micromol/L on existing management.